0075 How about an application?
Consider the reaction of hydrogen and oxygen molecules.
Here is a picture of the reaction in terms of the agencies of science and of natural philosophy.
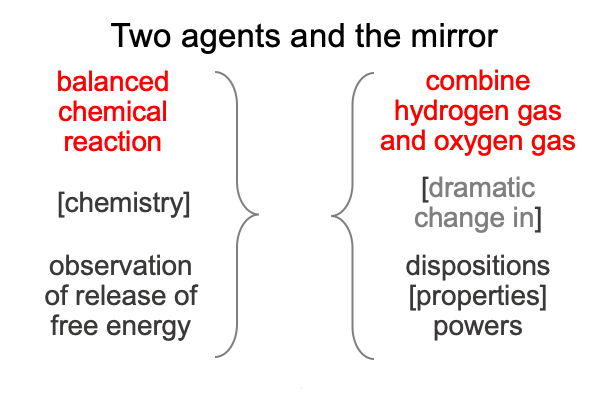
0076 These are very similar, so I do not anticipate many difficulties.
The reaction itself may be expressed as a hylomorphe, as an expression of Peirce’s secondness, as scientific process, or as a witness-able event.
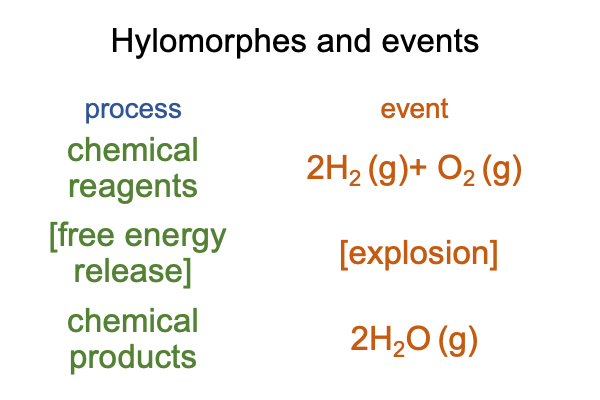
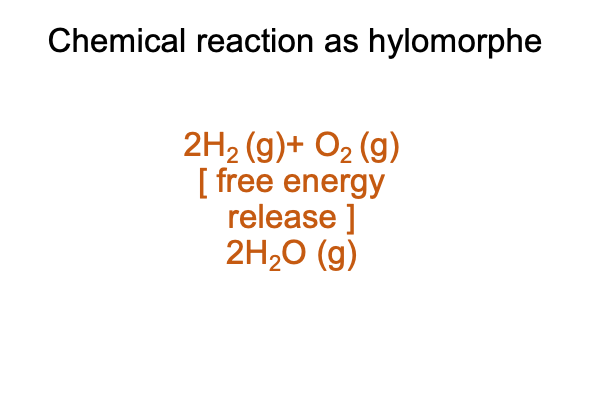
0077 The materially balanced chemical reaction may be structured as a hylomorphe. The contiguity may be described as a loss of free energy. This free-energy release makes the reaction spontaneous.
Here is a picture.
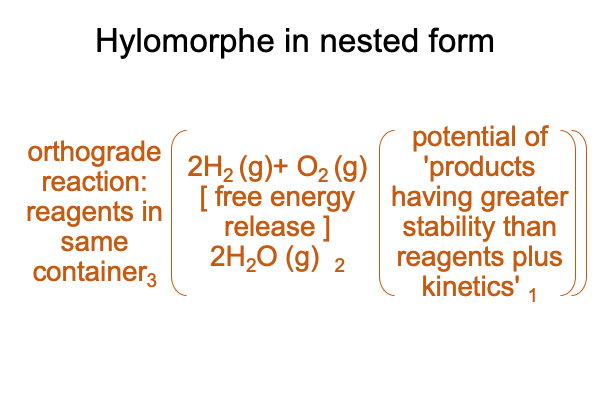
0078 A container loaded with hydrogen and oxygen gases will not explode until a spark starts the reaction. There is a significant “barrier” to reaction. Once that barrier is breached, then all hell breaks loose.
In order to include the kinetic barrier, I can add an item to the list of potentials in the following category-based nested forms.
0079 Chapter one of Tabaczek’s book, Emergence, discusses the central dogma of emergence.
First, the causes of emergent phenomena do not add up.
And second, they seem to.
0080 For example, the above orthograde reaction has been well studied and its mechanism (composed of modern truncated material and efficient causes) is well established.
0081 What happens when I use this reaction in a hydrogen-oxygen fuel cell?
A hydrogen-fuel cell may serve as a case study for emergent phenomena.
Sure, the hydrogen-fuel cell is designed by humans, but that is okay, because it has what emergent natural phenomena have, a life of its own.
0082 The spontaneous explosive reaction can be “tamed” (okay, “exploited”, is a better word) by separating molecular hydrogen’s disposition to give electrons and molecular oxygen’s disposition to receive electrons. All I have to do is find the right metal for two separated electrodes in water, where one electrode contacts H2 (g) and the other electrode contacts O2 (g). The gases are funneled in by tubing from nearby gas cannisters.
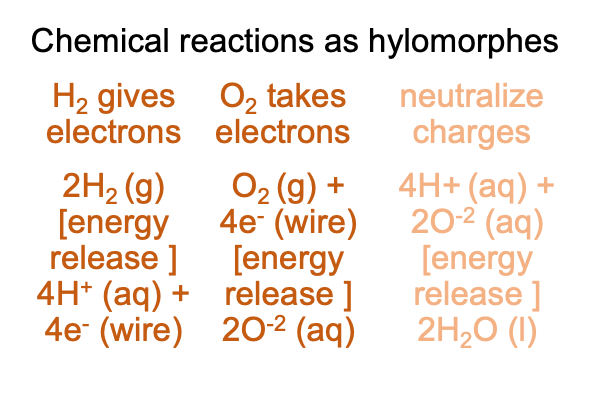
0083 Now, I label this separation of the oxidation (the electron-giving) and the reduction (the electron taking) sides of the original chemical reaction, “contragrade”, because it re-directs the spontaneous release of free energy into an electric current that can do work.
Here is a picture of the circuit.
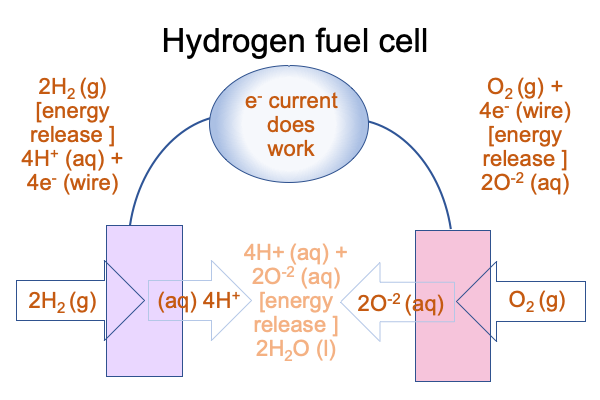
0084 At the anode, two hydrogen molecules touch the metal and separate into four hydrogen atoms which then release (because they are disposed to) their electrons into the electrode in order to become four aqueous hydrogen ions. Hydrogen ions love water and will make the solution acidic.
At the cathode, one oxygen molecule touches the metal and separates into two atoms that will take on two electrons each. They become ions that immediately attract hydrogen from nearby water molecules, resulting in four hydroxide ions. For simplicity, I write that the result is O-2 (aq). Hydroxide makes the water basic.
To complete the circuit, the hydrogen ions and hydroxide ions recombine to form neutral water somewhere between the electrodes.
0085 To improve this setup, an engineer places a matrix of polysulfonate in the water between the cathode and anode. Polysulfonate consists of sulfate ions covalently bonded to a carbon polymer, creating a forest of negative sulfate ions for the positive hydrogen ions to enter (for one side) and a ready supply of hydrogen ions that float out to neutralize hydroxide (on the other side).
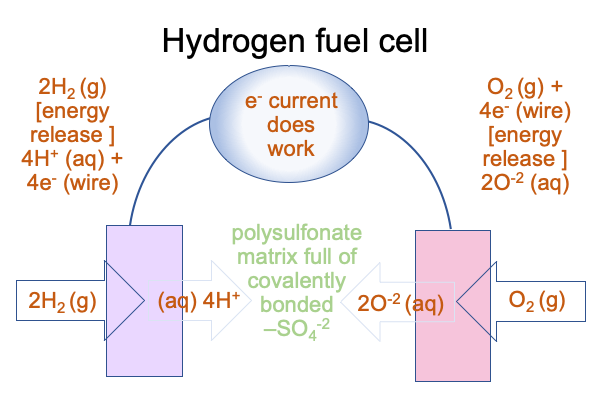
0086 At this point, I can draw several conclusions.
First, the contragrade (fuel cell) reaction does not add up to the orthograde original, because the hydrogen and oxygen gases do not mix and explode. Instead, a current is produced. This current is capable of, say, running a little motor. The contragrade set-up exploits the orthograde reaction by re-directing some (but not all) of the thermodynamic free energy released by the original (orthograde) reaction.
Second, the contragrade processes associated with this fuel-cell, as an emergent phenomenon, are novel. They would not exist except for the dispositions and powers of molecular hydrogen and oxygen. Plus, the homeodynamics of the contragrade processes can be optimized. For example, the polysulfonate matrix assists in the ability of aqueous acid and base to recombine.
Third, the energy captured by homeodynamics is dissipated when the current does work. So, the fuel-cell and its motor are morphodynamic (where “morpho” means “arrangement”). The way the motor relies on the fuel cell in such a way that a failure of the fuel cell will stop the power to the motor. In terms of the evolution of motors that use hydrogen-fuel cells, I suppose that means that machines with poor morphodynamics do not survive.
Fourth, the motor does not stand alone. It will be part of a larger machine. This introduces the concept of teleodynamics. The fuel-cell driven motor has material, efficient, formal and final causes.
