0185 Part 1, “The Phenomenon of Emergence”, covers the first third of Divine Action and Emergence.
Chapter one is titled, “The Science and Metaphysics of Emergence”.
0186 Emergent phenomena are classified according to order.
In first order phenomena, content includes properties that come into play as the content is situated.
0187 For example, water… I mean… H2O acts as a sticky dipole. That sticky dipole supports the situational or “bulk liquid” properties of water, including surface tension, high boiling point, and so on.
0188 Now, I explain.
Oxygen has 8 protons. An oxygen atom will take two electrons to own 10 electrons. Why does it want 10 electrons? Quantum mechanics explains. Electrons are waves. Around the positive point source of an atomic nucleus, they form standing waves. Standing waves may be described by geometric mathematical models. The lowest energy orbital, the one closest to the oxygen nucleus, is spherical and holds two “magnetically paired” electrons (1s(2)). The next energy orbitals include 1 “2s” or spherical and three angular node-bearing or “2p” orbitals, which may “hybridize” into four 2sp3orbitals.
0189 So, the math goes like this.
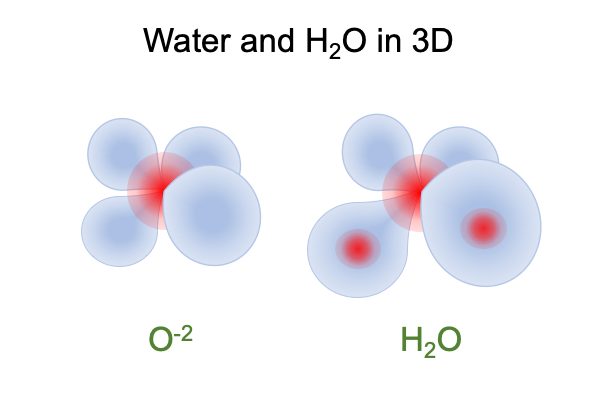
Oxygen has 8 protons. Oxygen has 1 lowest energy and 4 second to lowest energy orbitals. Each orbital can hold 2 electrons. When an oxygen atom has the power to fill all its orbitals with electrons, it becomes an O-2 ion because it owns 10 electrons (1s(2) and 2sp3(8)). The property is nice because the 8 positive proton nucleus is uniformly surrounded by 10 negative electrons in a mathematically symmetric arrangement.
0190 The problem?
O-2 is negatively charged. It is a beacon for positive charges, such as hydrogen ions, H+1, who have no electrons. Hydrogen ions are protons who cannot hold onto their electrons. So, they “share” electrons with other atoms, simply by burrowing into the electron-densities of one of those mathematically modeled orbitals. The electron orbital adjusts to form a “covalent” bond. A “covalent” bond is a mathematically modeled wave between two positive nuclei. When two electrons occupy the covalent wave, then each positive nucleus is held in place by what it perceives as a cloud of negative charge.
The result is neutral H2O, pictured above.
0191 H2O is a dipole. It has a positively charged side and a negatively charge side. So, gaseous water molecules will orient themselves according to an electric field. Plus, in liquid water they will tend to arrange themselves so that the positive side of one molecule is facing the negative side of another molecule.
Plus, in liquid water, each hydrogen ion loves to play a game of guessing which 2sp3 orbital it belongs to. When an electron cloud from molecule A comes close to a hydrogen-bearing orbital of molecule B, the hydrogen nucleus on B moves in response to the electron cloud in A, creating a temporary weak “hydrogen bond” between molecules A and B.
Here is a picture.
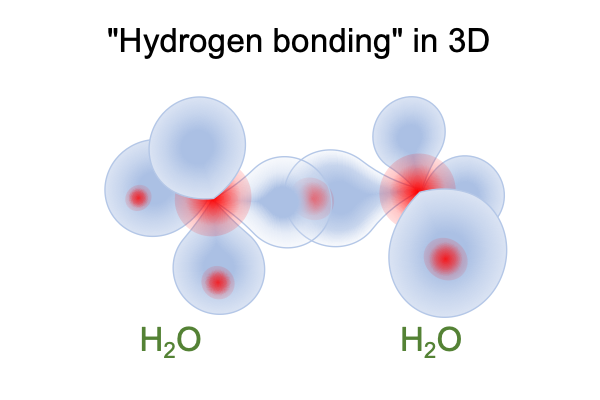
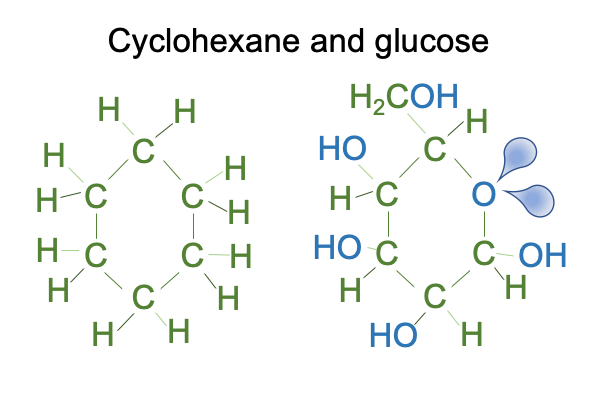
0192 Hydrogen bonding is water’s disposition, giving it the power to pull some molecules into bulk solution and to push some molecules out of bulk solution. If a molecule, such as cyclohexane, cannot play the game because all its hydrogen are tightly bound to carbon, then out it goes. When a molecule with basically the same carbon structure is loaded with covalently bound oxygen, it goes right into solution. Here is a picture of cyclohexane and glucose.
0193 Yes, I can describe what happens in terms of dispositions [properties] powers.

0194 Plus, I can portray the bulk property of glucose solubility as the situation level of a sensible construction.
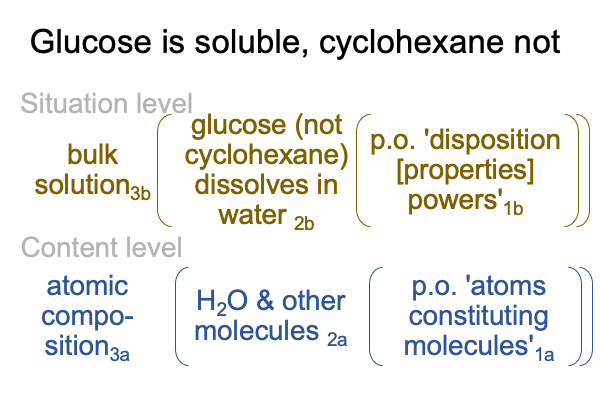
0195 So, I can say that the fact that glucose dissolves in water but cyclohexane does not is sort of like a first order emergent phenomena, based on the hydrogen bonding of water. Other bulk properties of water, such as its remarkable surface tension, belong to the list of first-order emergent phenomena.
0196 In order to get to second order emergent phenomena, I need to add a twist.
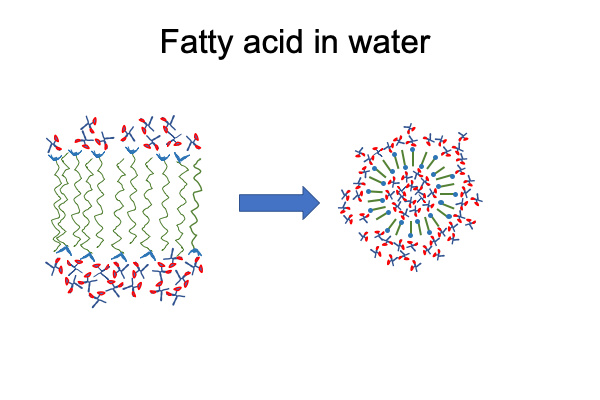
How about a fatty acid entering bulk water?
Here are classical chemical portraits of these two molecules. I color code the carbon, oxygen and hydrogen for better visualization. Notice that the hydrogen bound to carbon are… well… just like the hydrogen in cyclohexane. They are not available for hydrogen bonding. But, like molecular hydrogen, they are disposed to vigorously reacting with molecular oxygen, which is why laboratories in organic chemistry have fire extinguishers nearby.
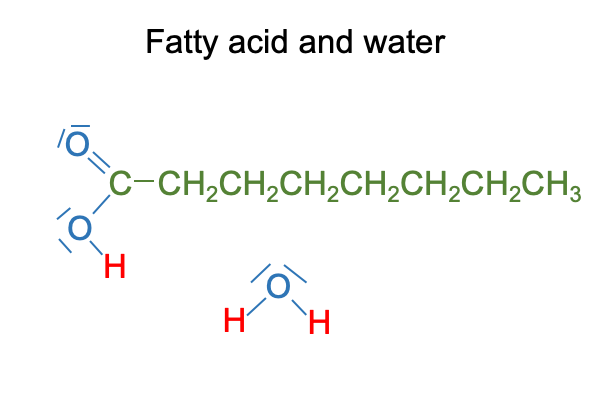
0197 So here is the conundrum.
Water wants to hydrogen bond to the carboxyl group and pull it into solution. At the same time, water wants to form a cage around the long alkane tail and drive it out of solution.
So what happens?
Fatty acids will form a bilayer. The alkane-tails collect in the middle, excluded by hydrogen bonding. Water-facing carboxyls are pulled into solution by hydrogen bonding.
When the bilayer curves, it produces an “inside” and an “outside”, resulting in a micelle.