0247 Let me start with the reagents of glucose and oxygen. Typically, glucose is taken by a cell out of the bloodstream through special pathways. Oxygen is breathed in as gas, then attaches to hemoglobin in the lungs, then is released from hemoglobin into capillary tissue. How crazy is that? The transport of oxygen is another special pathway.
These pathways work because of the properties of glucose and oxygen. Here, the technical term, “properties” labels the contiguity between dispositions and powers. As such, properties are not real elements, they are contiguities between two real elements. Disposition is what a thing tends to do. Power is what a thing is capable of doing. These two real elements will change, depending on the normal context3 and potential1, but they always contiguous. Each pair of disposition and power displays the contiguity called “property”.
0248 Here are two properties of glucose and oxygen.
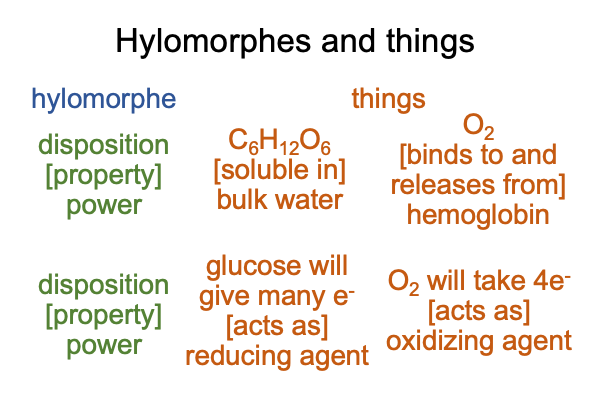
These hylomorphes describe how glucose and oxygen find paths to the mitochondria. The mitochondria is where the biochemistry of respiration occurs. The lungs are where oxygen bind to hemoglobin. The capillaries are where oxygen lets go of hemoglobin. Since glucose is soluble in water, it can float in the plasma of the blood. The concentration of glucose is tightly regulated, because there are other things that can feed off glucose, such as bacteria. Too much glucose in the blood invites infections.
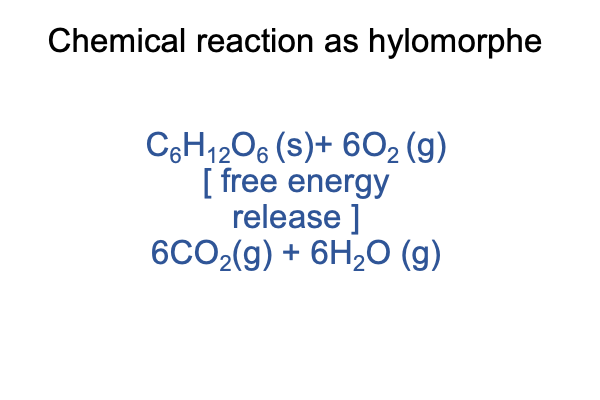
These hylomorphes also describe how one orthograde (or spontaneous) chemical reaction will get split, by mitochondria and the cell, into two.
Here is the one reaction.
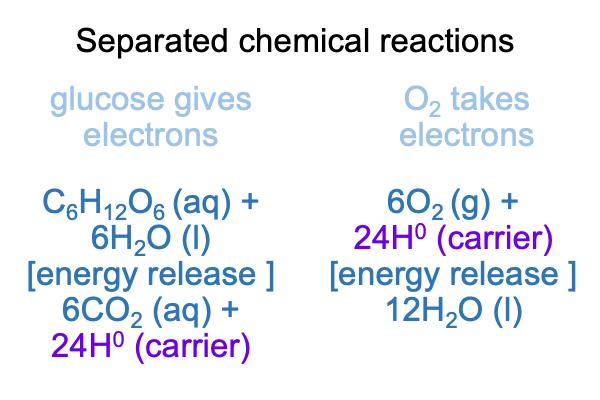
0249 How does the one chemical reactions get split into two? Glucose releases electrons. Then, these electrons are transported by specialized molecules (sometimes with the hydrogen ion tagging along). Where are the electrons (e-1) and hydrogen atoms (a H+1 and an e-1, that is, a H0) transported? The are carried to a system where oxygen takes electrons to form water.
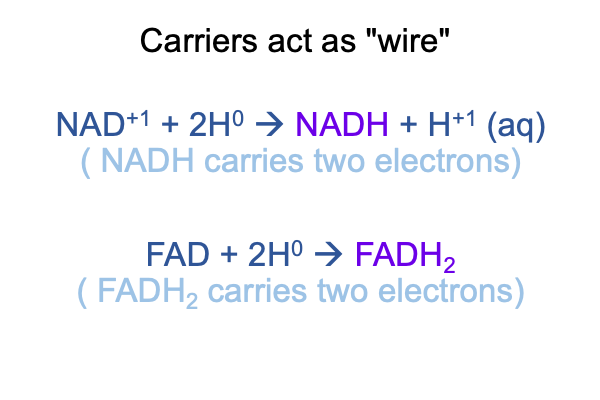
0250 Here are labels for the carriers. The labels NAD+ and FAD are acronyms for rather exotic biochemical names. I will pass over these details, because I know that these molecules have the disposition to take on electrons (e-1) as well as to release electrons (e-1). Sometimes, hydrogen ions (H+1) are along for the ride (hence the term, H0). These biomolecules have the power to transport high-energy electrons.