0302 In section 5.5 of Semiotic Agency and sections 9.4 and 9.5 of Pathways, Alexei Sharov presents a replicator-niche coupling model. Several items are required: water, oil, pigments (in oil), light, and two complementary molecules that are separate in water, yet combine to form an active site when they attach to the surface of an oil droplet.
0304 Here is a picture.
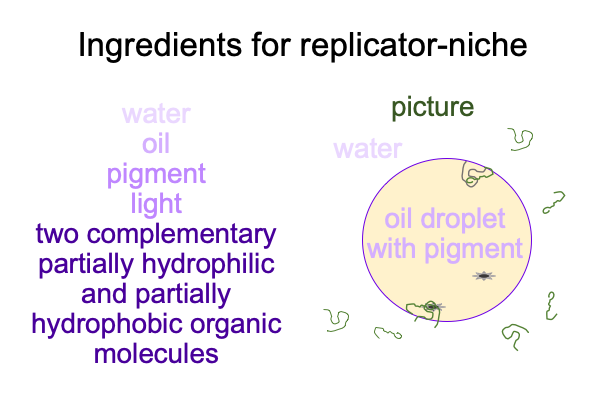
0305 Let me start with the oil droplet.
Water tends to drive alkanes out of solution. That is why alkanes form oil droplets in water. These droplets are not really stable, because they are not held together because of mutual attraction, but are held in place by the fact that each water molecule networks with other water molecules so well that, if a molecule does not participate in water’s hydrogen-bond networks, it gets driven out of solution. That also applies to the pigment, which is oil-soluble and not water-soluble.
0306 What about the polymers?
Parts of complementary polymers are soluble in water. Other parts are not as soluble. So, parts are driven out of water and parts are pulled back into water. These molecules collect on the surface of the oil droplet, then couple with one another, with the pigment and with an alkane, which is part of the oil droplet.
0307 There are no oxygen molecules in the picture. Today, the Earth’s atmosphere is around 20% oxygen and 80% nitrogen. In the early Earth’s atmosphere, reduced carbon compounds make the smaller fraction and nitrogen makes the large fraction. More or less. No scientist can go back in time and measure the composition of the atmosphere of the early Earth.
Reduced carbon in the atmosphere goes with the alkanes in the oil. Much of the light of the early sun is absorbed by the carbon-rich atmosphere, but some makes it down to pigments in the oil droplet. The pigment and complementary polymers conjoin in two locations in the figure below.
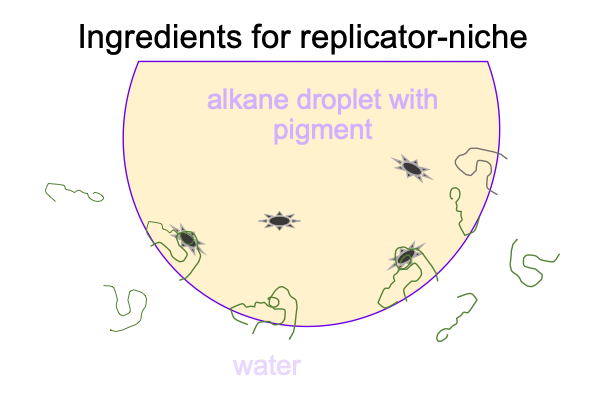
0308 Then, what happens?
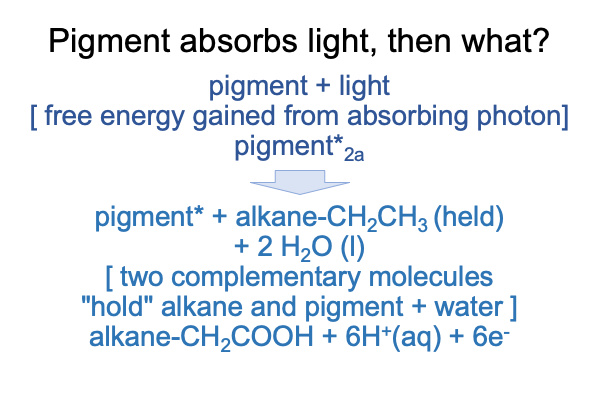
The pigment absorbs a photon and becomes an electronically excited pigment.
Then, the energy captured by this pigment initiates a chemical reaction, where the alkane is oxidized to a fatty acid. Oxidation releases electrons. One among many possible oxidations is pictured above. With a little more oomph, that carboxylic acid would pop off as carbon dioxide. However, this reaction stops as an alkane chain with a carboxylic acid at the terminus. I call this molecule a “fatty acid”.
In the following figure, the two processes are depicted as two dyads. Each dyad exhibits the structure of reagents [turn into] products.
0309 Now, theoretically a reduction reaction is close at hand. If the oil droplet is near a chemical that can accept the electrons, then a coordinated reduction can take place. For example, the hydrogen ions and the electrons can combine to form 3H2(g). Or, atmosphere nitrogen (N2) can be reduced to ammonia, 2NH3.
