Looking at Slavoj Zizek’s Book (2024) “Christian Atheism” (Part 8 of 33)
0090 By the time that I get to Zizek’s question whether God believes in Himself, I have already crashed into the guardrails of Marxist materialism, Hegel’s phenomenology of thesis, antithesis and synthesis, and Lacan rescuing Freud. Yet, I feel that this examination stays oriented to Zizek’s parallaxical title, “Christian Atheism”. After all, at this point, who knows whether my diagrams belong to Peirce’s philosophy or scholastic theological speculation?
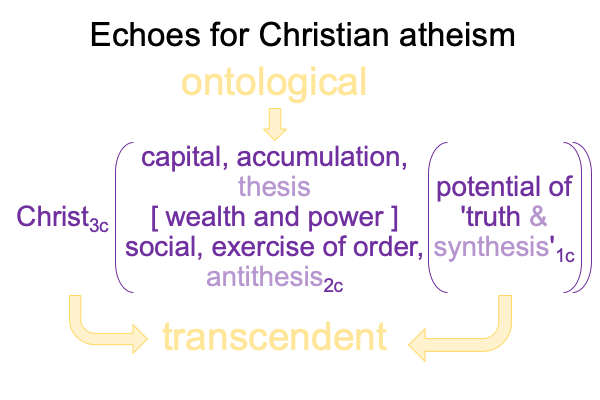
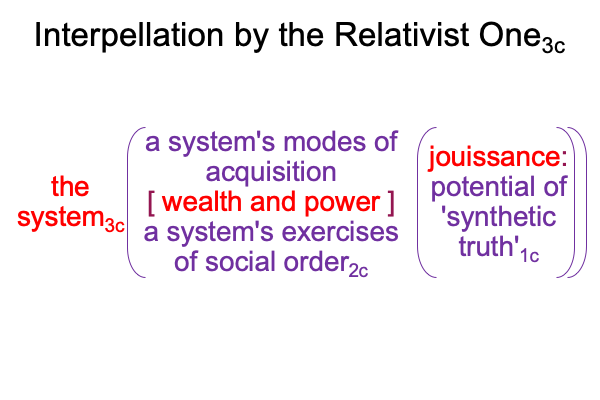
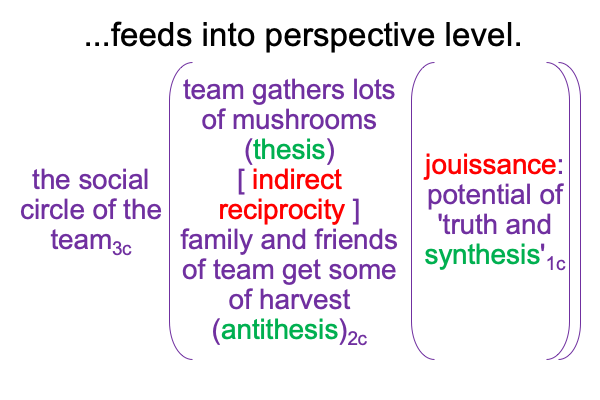
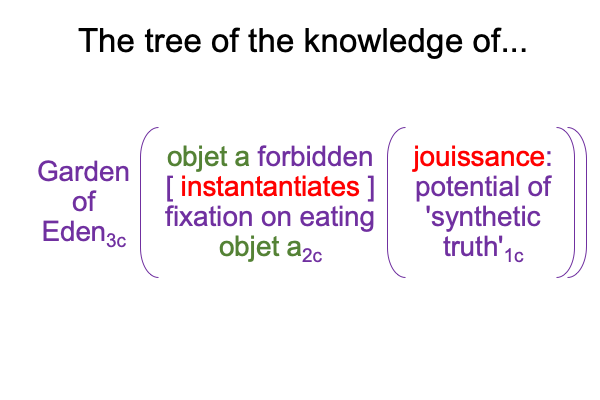
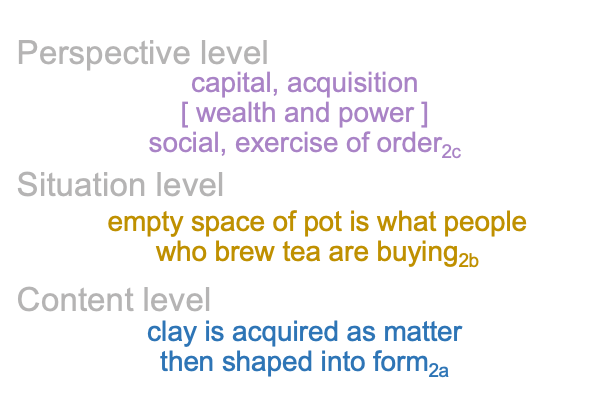
A sublime synthesis1c, coincides with the possibility of truth1c, and sustains the actuality2c of a thesis2c and its antithesis2c. The [contiguity] between the two real… er… ontological elements2c, sustains the continued constellation of a transcendent normal context3, that proclaims, “This category-based nested form is alive.”
This category-based nested form illustrates what Freud calls, “the death drive”.
Through repetition, obsession, sublimation and fatalism, the normal context of the Holy Spirit3 operates on the potential oneness of God1, that is, the Islamic Allah1.
Say what?
If God is great, then God is alive. And, if God is alive, then God manifests in all three of Peirce’s categories. The Oneness of God1 potentiates the dyadic actuality of His Own Actualization2 in the normal context of His Own Spiritual Being3.
0091 Here is a picture of One God, constellating the Three Persons as well as the Sublime thesis, antithesis and synthesis.
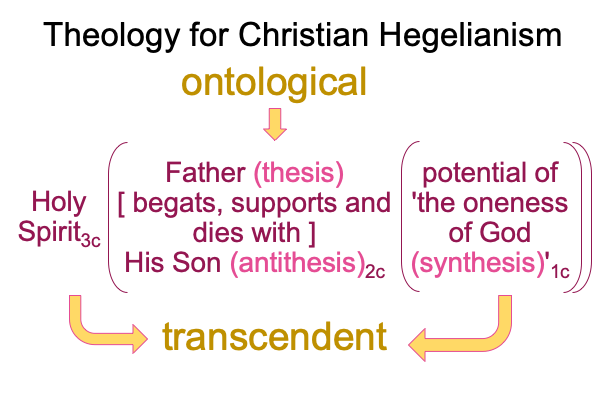
0092 Weirdly, an always postponed synthesis1c, undergirds the actuality of {the thesis of the Father (corresponding to the Old Testament) [in contiguity with] the antithesis of the Son (corresponding to the New Testament)}2c. In other words, a sublime synthesis1c stands under the ontological realness of a dyadic actuality2c, containing the tension that is the first Person of God [in human relation with] the second Person of God2c, in the normal context of a third, transcendent Person of God3c, Divine Inspiration3c.
To which the highly academically credentialed purveyor of oversimplification replies, “God is [love].”
How so?
The historic contiguity, [begets, supports and dies with], represents a human connectivity… er… contiguity… that some would label as [love].
Oh, it is that parallax business again!
To me, the category-based nested form pictured above is in sync with Lacan, and Zizek is on the verge of revelation.
And maybe, I am on the verge of consternation.
0093 The neurotic cannot sustain the tension. The neurotic wants to reify the contiguity and say, “God is love and ‘love’ is whatever I want the label to signify!”
The pervert aims to sell a product, labeled “The Love of God”, to the neurotic.
After all, isn’t that what the neurotic desires?
The neurotic wants thesis to precede antithesis then antithesis to resolve into a synthesis that the neurotic can… um… label.
But, what if the synthesis never arrives? What if the synthesis is constantly postponed, because a sublime synthesis1supports the ontological realness of thesis [in tension with] antithesis2, and the dyad2 is contextualized by a transcendent mediator3, a person3, a normal context3 that is… what other word can I use?… alive.
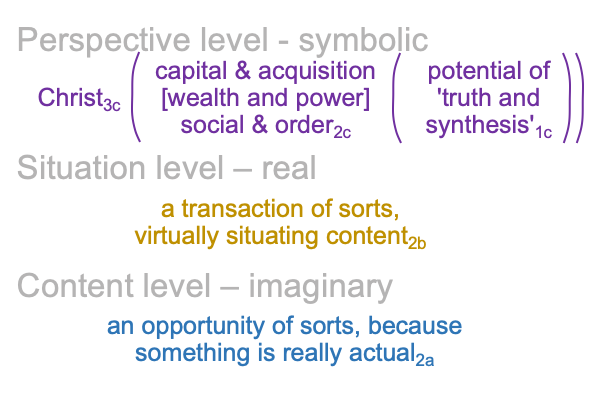
0094 Note that there are two sets of three labels in the single living relational being depicted above.
0095 The first set belongs to thirdness and secondness, normal context3c and actuality2c, and corresponds to the Trinity, Three Persons in One God. The three labels are Holy Spirit, Father and Son. The relational nature intrinsic to the Trinity may be formulated in many ways. The above figure is developed in the second interlude in How To Define the Word “Religion”, by Razie Mah, available at smashwords and other e-book venues.
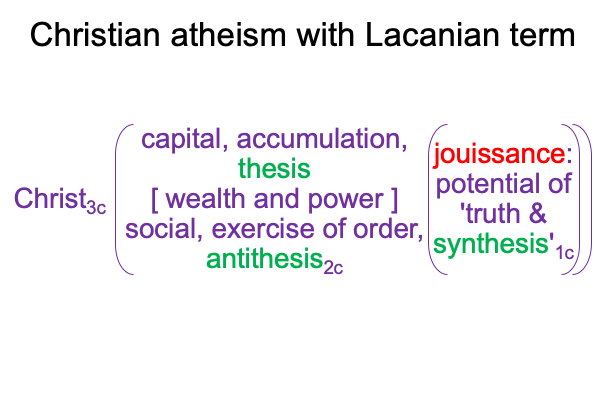
From the Trinity, Zizek chooses his own Person3 (Christ3c) to be the One3 for his theoretical configuration.
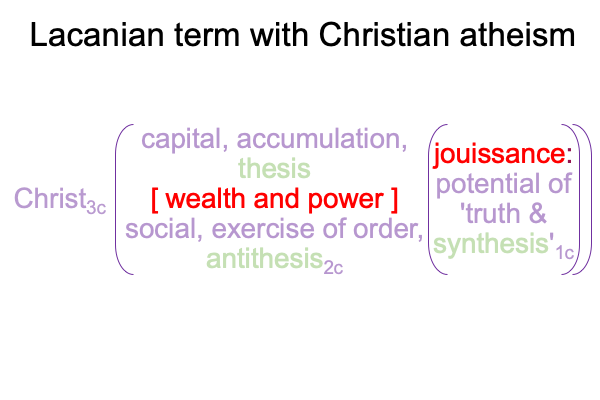
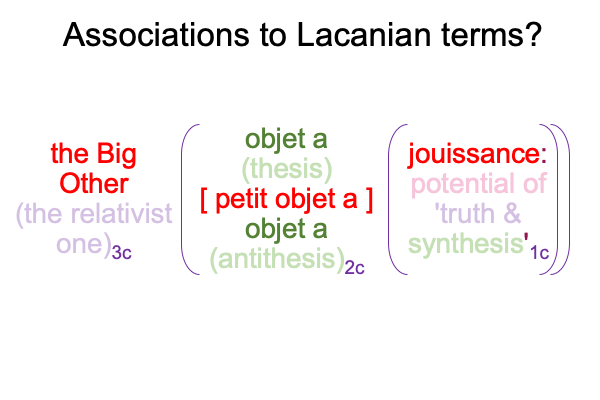
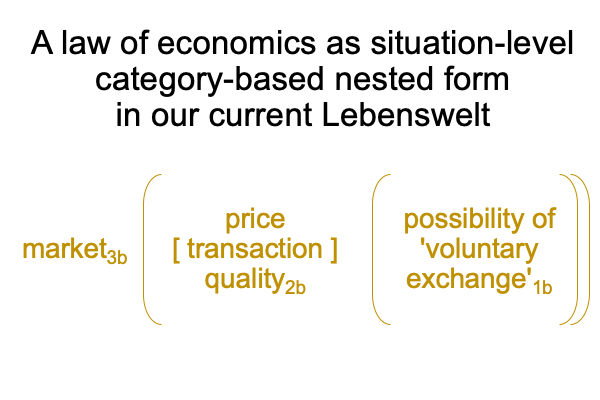
0096 The second set belongs to secondness and firstness, actuality2 and possibility1, and corresponds to Hegel’s three standings: thesis as matter2, antithesis as form2, and synthesis as the potential of matter [substance] form1. In these standings, the ontological2 emerges from and situates the sublime1… er…. transcendent.
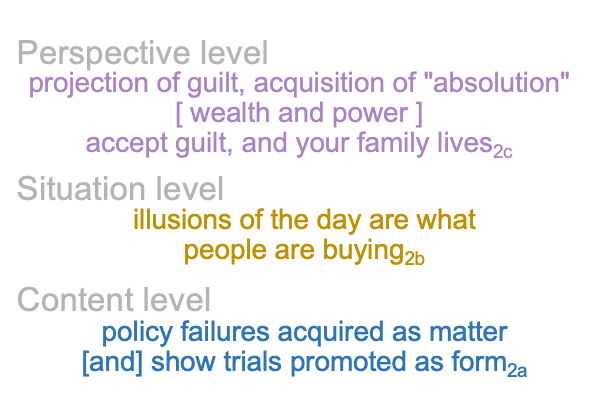
Here is where the atheist pursues apparent advantage, by asking, “What kind of evil Father would do something so stupid as to sacrifice his own Son in order to redeem the same babbling morons that He tried to destroy in Noah’s flood?”
In a way, this question is purely materialist, because it blurs the gap between actuality2 and possibility1. Why would God allow Himself to die? Would dying destroy His Oneness? Or would it forever confound the question of God’s oneness with the reality of God’s Self-Actualization?
Zizek poses a similar question. Does God3c believe in Himself2c, as a thesis2? Perhaps, the Holy Spirit3c does. But the Father’s Son2c has a moment of concern. In asking this question, the author relies on a specialized language, a Hegelian language, where he can talk about God2c, as a thesis in [contiguity] with its antithesis, emerging from (and situating) a synthesis, the oneness of God1c.
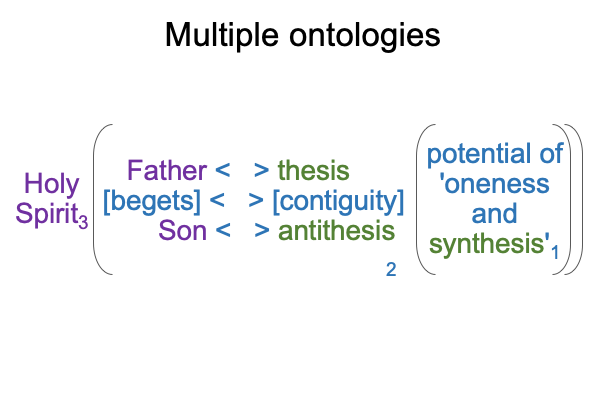
0097 I suppose that a similar purely relational structure applies to my own ontology and transcendence.
For the example, in Freud’s terms, my “superego” might go with thesis (and matter) and my “ego” might go with antithesis (and form). So, I have a busybody upstairs always telling me the right thing to do. Who doesn’t? But, what is worse is the synthesis1, which dwells in the realm of possibility because (heaven forbid!) I3 would never act upon the ideas1 undergirding the tension between me telling me the right thing to do [and] me making excuses for why I cannot do the right thing2. I dare not name those desires1 without the assistance of psychoanalysis within the seclusion of the analytic dyad.
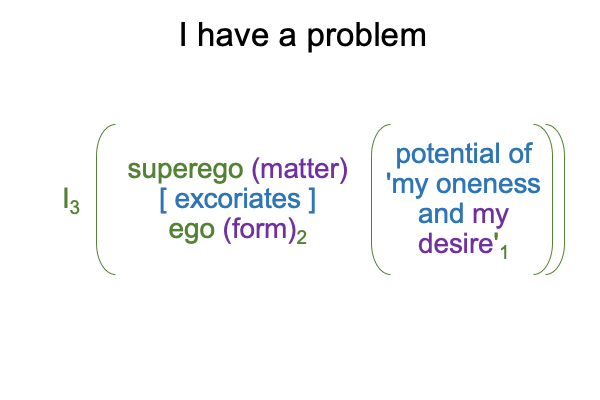
Consequently, I tell my psychotherapist that I have a problem with making excuses.
And, I hope that the doctor does not reify my desire1 and write a diagnosis2 in a database3 that can be hacked and sold to whoever wants to pay for that type of information.